Abstract
Introduction : Multiple myeloma (MM) is a hematologic cancer of the plasma cells, which are found in the bone marrow. The early symptoms of MM are often vague and non-specific, such as fatigue, bone pain and back pain. These vague symptoms lead patients to present first to their primary care physician and often delay diagnosis resulting in progressive disease [MayoClinic.org]. The median time for MM diagnosis (163 days) is longer than for most other hematologic cancers including leukemias and lymphomas [Howell et al, BMC Hematol, 2013]. Delayed diagnosis of MM is commonly associated with the development of chronic complications such as renal impairment, infections, neurological disease, bone disease and anemia [Kariyawasan et al, Q J Med, 2007]. Therefore, early detection and diagnosis of MM are essential to avoid downstream complications, and may significantly reduce the treatment costs associated with these complications. In the assessment of symptomatic patients, National Comprehensive Cancer Network (NCCN) practice guidelines recommend using three tests to maximize sensitivity and specificity: serum protein electrophoresis (SPE), serum immunofixation electrophoresis (SIFE) and serum free light chains (SFLC) [NCCN Multiple Myeloma Guidelines Version 3.2017]. However, physician compliance with these guidelines is hampered by the many different test options available and the low visibility of Myeloma on the list of possible causes for the primary care physician. In the current study, we modeled the effect of converting a hospital system over to guideline compliance in testing for Multiple Myeloma.
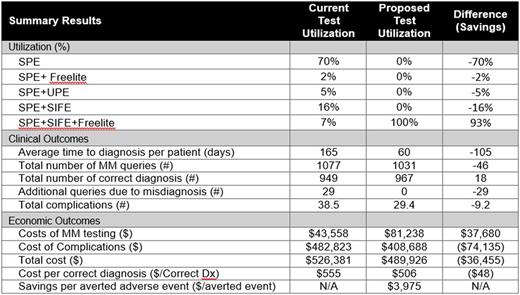
Methods : A decision analytic model was developed to estimate the total costs and annual budget impact when using the guideline recommended panel for early diagnosis of MM patients. Using a US hospital (i.e., integrated delivery network [IDN]) model perspective, a decision algorithm was developed based on NCCN and IMWG clinical practice guidelines and further validated by clinicians [Rajkumar et al, Lancet Oncol. 2014]. Comparators include SPE alone, SPE+SFLC, SPE+UPE (urine protein electrophoresis), SPE+SIFE and SPE+SFLC+SIFE. Epidemiology estimates were from the SEER database [Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat]. Test sensitivity and specificity and downstream complication rates were based on published sources [Katzmann et al, Clin Biochem Rev, 2009 and McTaggart et al. Am J Clin Pathol, 2013]. Lab and imaging costs were collected from the CMS laboratory fee schedule and downstream complication treatment costs from the HCUP National Inpatient Sample database.
Results : For an average IDN, a total of 967 patients are estimated to undergo evaluation for MM each year. Conversion from typical current practice to guideline compliance is estimated to reduce the time to MM diagnosis from 159 days to 45 days per patient (64% reduction). The number of correct MM diagnoses increases from 949 to 967 per year. Hospital laboratories would expect to invest $37,680 per year in additional diagnostic costs in order to save the hospital system a total of $74,135 annually in averted complications (24% reduction in complications) resulting from earlier diagnosis. From a cost-benefit perspective, this translates to a reduced cost/correct diagnosis of about $48 and savings per averted complication of $3,975 for the hospital system.
Conclusions: Guideline recommended testing of patients with early symptoms of Multiple Myeloma can help reduce costs, improve patient outcomes and increase efficiency of care. Hematologists, as stewards of the guidelines, should help in educating primary care physicians on warning signs of MM and appropriate diagnostic tests.
Wu: Celator/Jazz: Consultancy. Jensen: Celator/Jazz: Consultancy. Cyr: Celator/Jazz: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal